Did Your Cartiva® Toe Implant Fail?
See If You Qualify For Financial Compensation
Let's begin with your Free Case Review
Patients who received a Cartiva® Toe Implant and experienced device failure leading to surgical repair, removal, or replacement, may be entitled to Compensation!
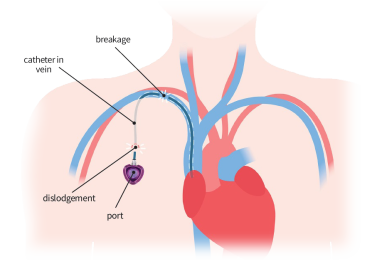
Patients and families of patients who received a Bard PowerPort implantable catheter and then suffered complications may be entitled to Significant Financial Compensation. Check now!



Patients who received an Implantable Catheter may be entitled to Significant Financial Compensation.


Confidential
Review
Secure
Site
Published Study Shows Alarming Failure Rates
By clicking ‘Do If I Qualify’ and submitting my request, I confirm that I have read and agree to the Privacy Policy and Terms & Conditions of this site and that I consent to receive emails, phone calls and/or text message offers and communications from Sheldon Law Group LLP at any telephone number or email address provided by me, including my wireless number if provided in the form of at least 2 SMS messaged per day with account alerts. I understand there may be a charge by my wireless carrier for such communications. I understand these communications may be generated using an autodialer, AI (Artificial Intelligence), may contain pre-recorded messages and that consent is not required to utilize Sheldon Law Group LLP’s services. I understand that this authorization overrides any previous registrations on a federal or state Do Not Call registry. Accurate information is required for a free evaluation. For Help at any time, text "HELP". To unsubscribe at any time, reply "STOP". Message & Data rates may apply.
According to a study published in October 2020 by the American Orthopedic Foot and Ankle Society*, the results show an alarming rate of device failure.
Study Highlights:
⇢ 64% of individuals who received a Cartiva implant for hallux rigidus experienced failure within four weeks of surgery.
⇢ 79% of individuals who received a Cartiva implant for hallux rigidus experienced failure 19 months after surgery.
⇢ The study also found that the implant did not preserve the joint space or prevent further cartilage damage.
Broken Promises, Painful Realities: Cartiva® Implant
Cartiva® synthetic cartilage implants were hailed as a revolutionary solution for patients experiencing hallux limitus or hallux rigidus, a form of arthritis that affects the first metatarsophalangeal joint, or big toe. This form of arthritis affects millions of people each year.
Cartiva® Toe Implants are synthetic devices that are supposed to relieve pain and improve mobility for people with arthritis in the big toe. However, instead of experiencing relief, many patients reported severe side effects such as:

✘ Infection✘ Revision Surgery✘ Fragmentation
✘ Bone Erosion✘ Nerve Damage✘ Loss of range of motion
Patients Report Premature Cartiva® Implant Failures
If your Cartiva® toe implant has failed, causing pain, limited mobility, and additional surgeries, you're not alone. Thousands of patients who received Cartiva® implants for hallux rigidus are complaining of
• Premature implant failure requiring revision surgery
• Persistent pain and discomfort despite revision surgery
• Loss of mobility and function in the toe
Some of these patients have filed lawsuits against the manufacturer of Cartiva Toe Implants, claiming that the company failed to warn them of the risks and defects of the product. These patients are making serious allegations against the company including:

• Defective design and materials: Studies suggest Cartiva® implants degrade and shrink, causing instability and pain.
• Fast-tracked FDA approval: Concerns exist that potential risks were not adequately assessed before market release.
• Failure to warn of risks: Patients and healthcare providers may not have been fully informed about the potential for implant failure.

If You Had to Have Revision Surgery After Receiving a Cartiva Toe Implant, We Can Help.
If you have suffered from complications after receiving a Cartiva Toe Implant, contact us now. We are fighting to protect the rights of patients allegedly injured by Cartiva toe implants and hold the device makers fully accountable for putting profits above the health and well-being of patients.
If you received a Cartiva toe implant and were forced to have revision surgery to remove or replace the device, you may be entitled to significant financial compensation. Contact us now to learn more about your rights and whether you are entitled to compensation.
Attorney Advertising Disclaimer: The information you obtain at this site is not, nor is it intended to be, legal advice. You should consult an attorney for advice regarding your individual situation. We invite you to contact us and welcome your calls, letters and electronic mail. Contacting us does not create an attorney-client relationship. Please do not send any confidential information to us until such time as an attorney-client relationship has been established. Prior results do not guarantee a similar outcome.
This website is intended to comply with California legal advertising rules. If you believe any content is inaccurate or misleading, please contact the firm HERE for clarification.
PAID LEGAL ADVERTISEMENT
1250 Connecticut Avenue NW, Suite 700, Washington, DC 20036
©Copyright 2026 Sheldon Law Group, LLP. All rights reserved.
